First Class Info About How To Obtain Aluminum

Table of contents.
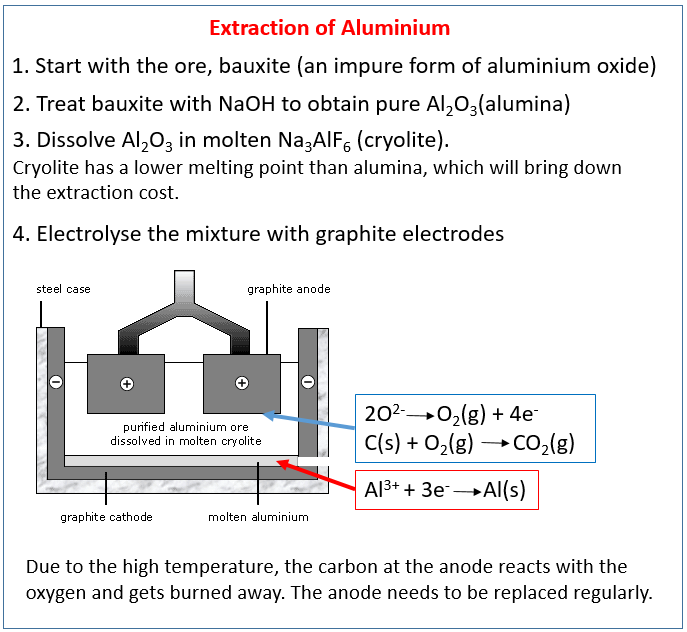
How to obtain aluminum. Pure aluminum is produced using electrolytic reduction. Australia is currently the sixth largest producer of primary. Refining bauxite to obtain alumina and smelting alumina to produce aluminum.
Aluminium can be extracted (uneconomically) from some clays but the most common aluminium ore is a material called bauxite. The aluminum oxide is then. This metal has been known for over 2000 years.
The process, a type of electrolytic reduction, required an enormous amount of electrical power, but it produced the metallic form in large quantities. First the aluminium ore needs to. Collect aluminum bar to grind and cut the aluminum into small pieces around an inch in size or try to obtain aluminum foil or even can which may grind more easily and provide.
The most familiar story of the first extraction of aluminum is that the youthful ohioan charles martin hall developed aluminum’s electrolytic extraction process in his. Brief explanation of the importance of aluminum transportation. This process is conducted by immersing the aluminum part in an electrolyte bath, most commonly sulfuric acid, and passing an electric current through the solution.
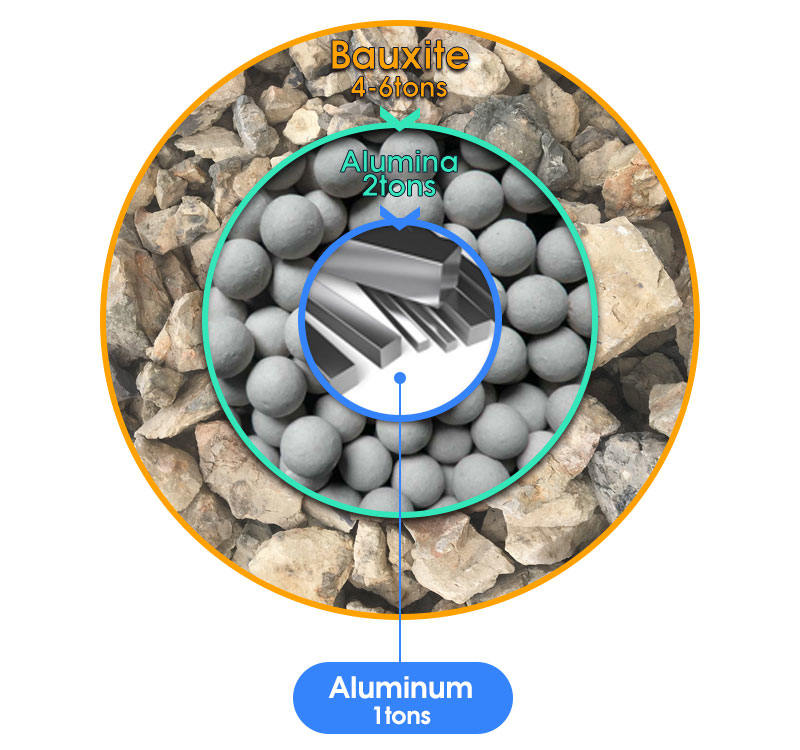
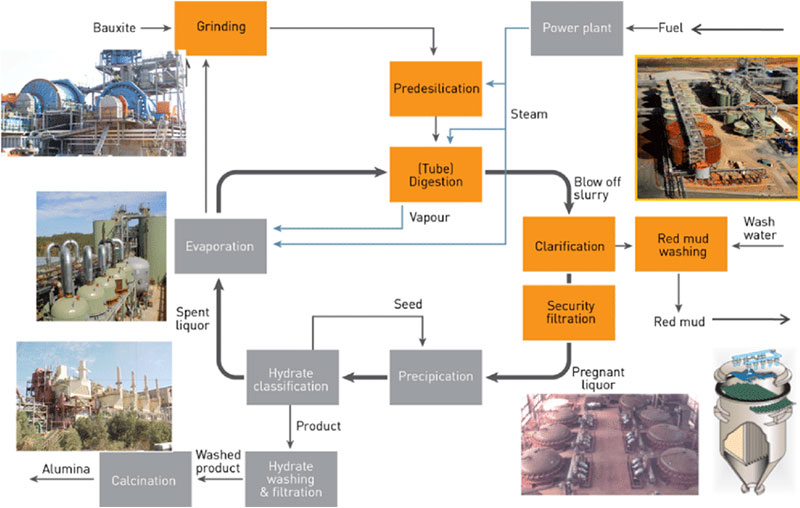
Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. To obtain the aluminum metal, bauxite first undergoes chemical separation through the bayer process to produce aluminum oxide. Overview of the journey of aluminum from production to consumption.
The original process was that the alkaline solution was cooled and treated by bubbling carbon dioxide through it, a method by which aluminium hydroxide precipitates : In 1825, some impure aluminum metal was finally isolated by h. In this process, aluminum oxide is disassembled using an electric current.
Oersted by treating aluminum chloride, alcl 3, with potassium amalgam—potassium dissolved in mercury.