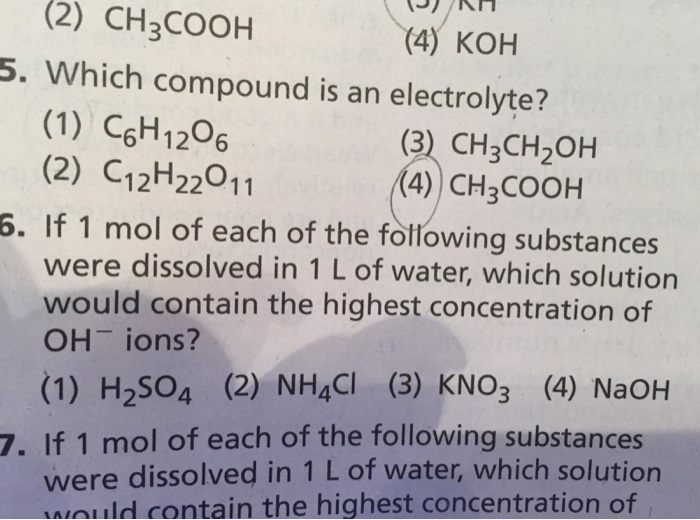Awe-Inspiring Examples Of Tips About How To Tell If A Compound Is An Electrolyte
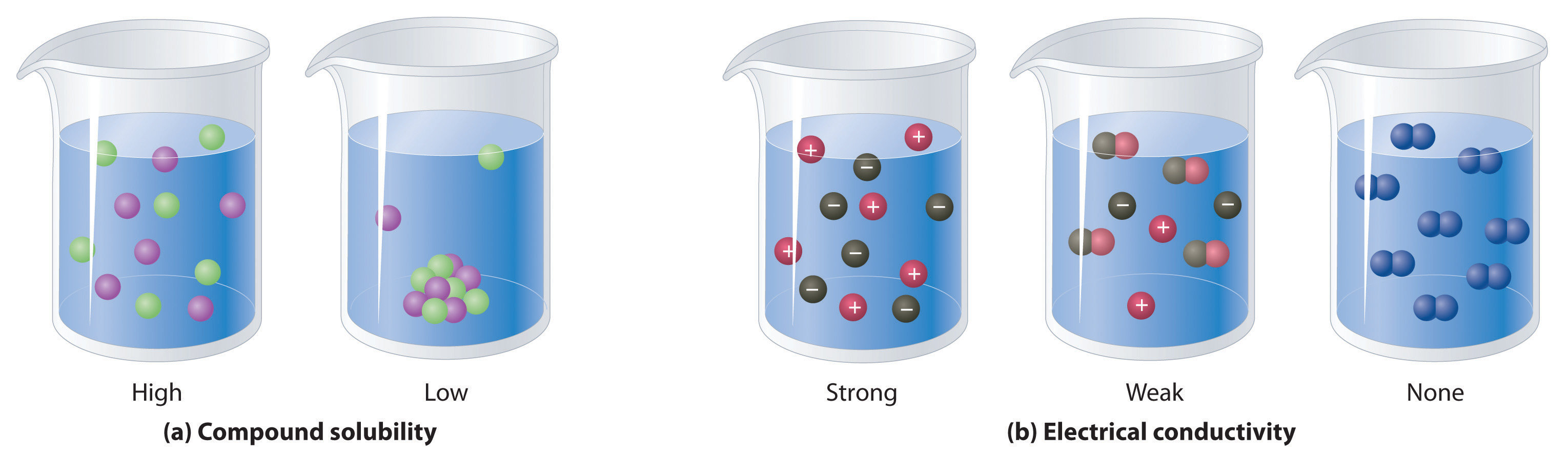
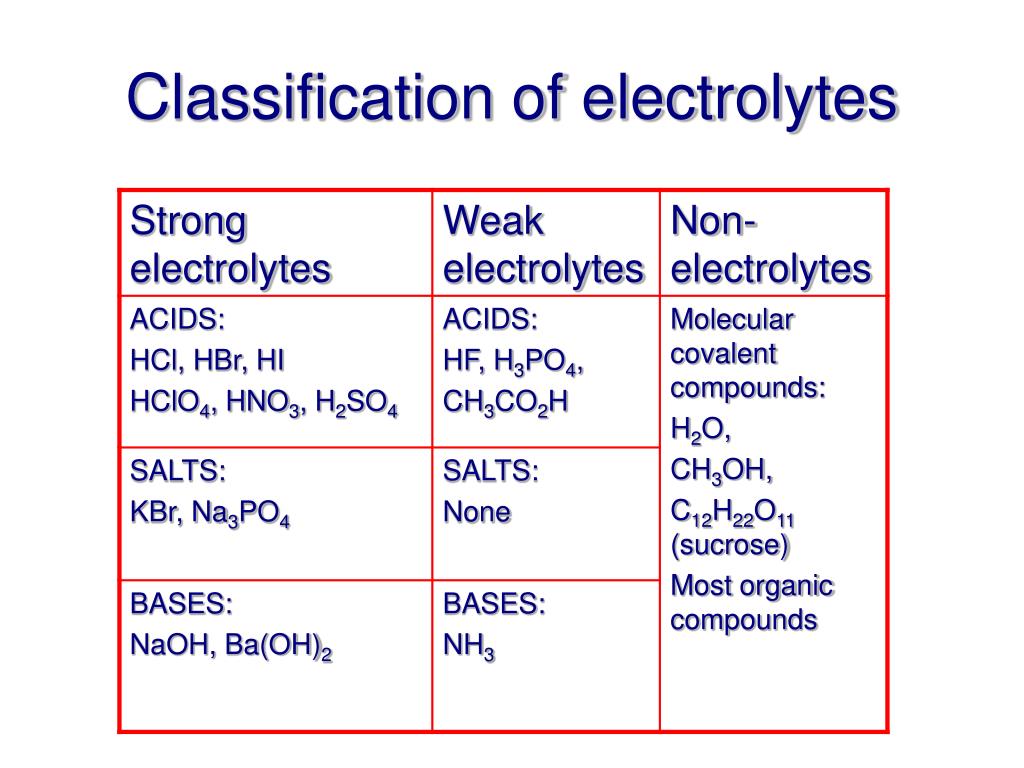
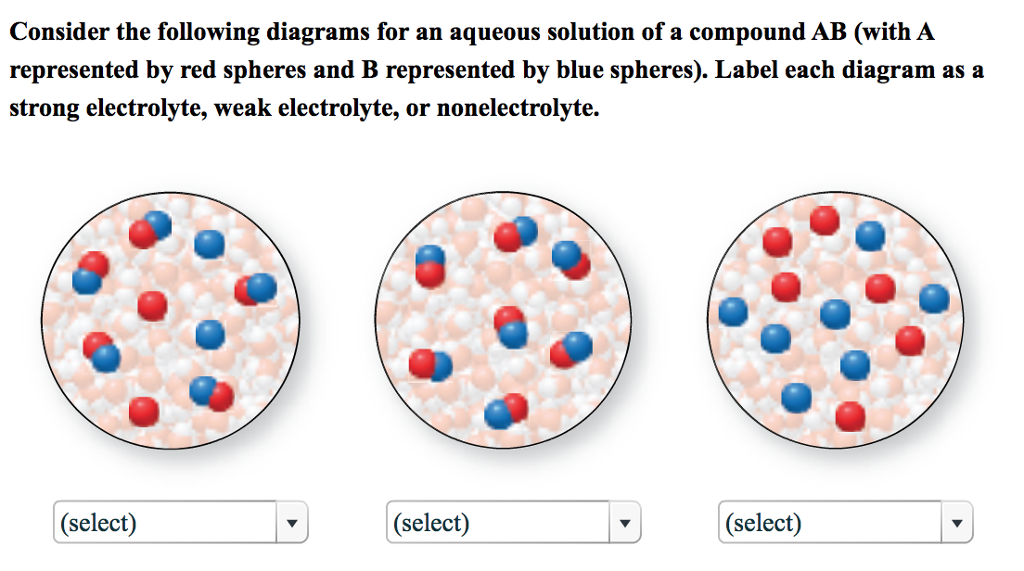
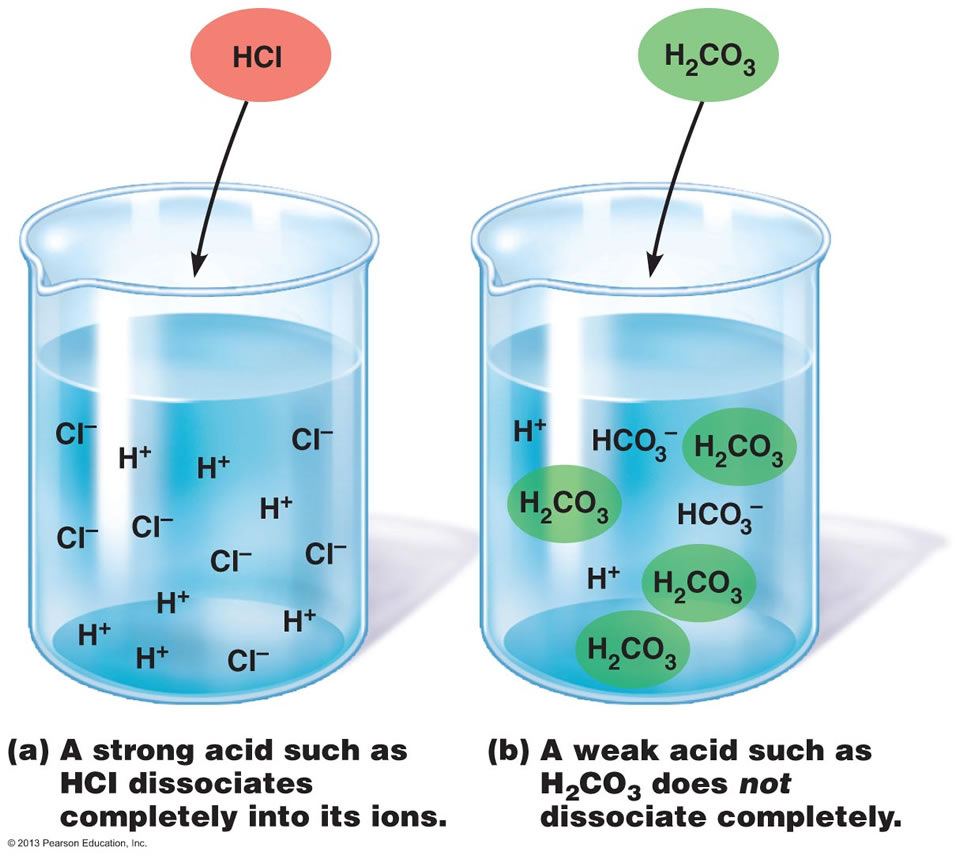
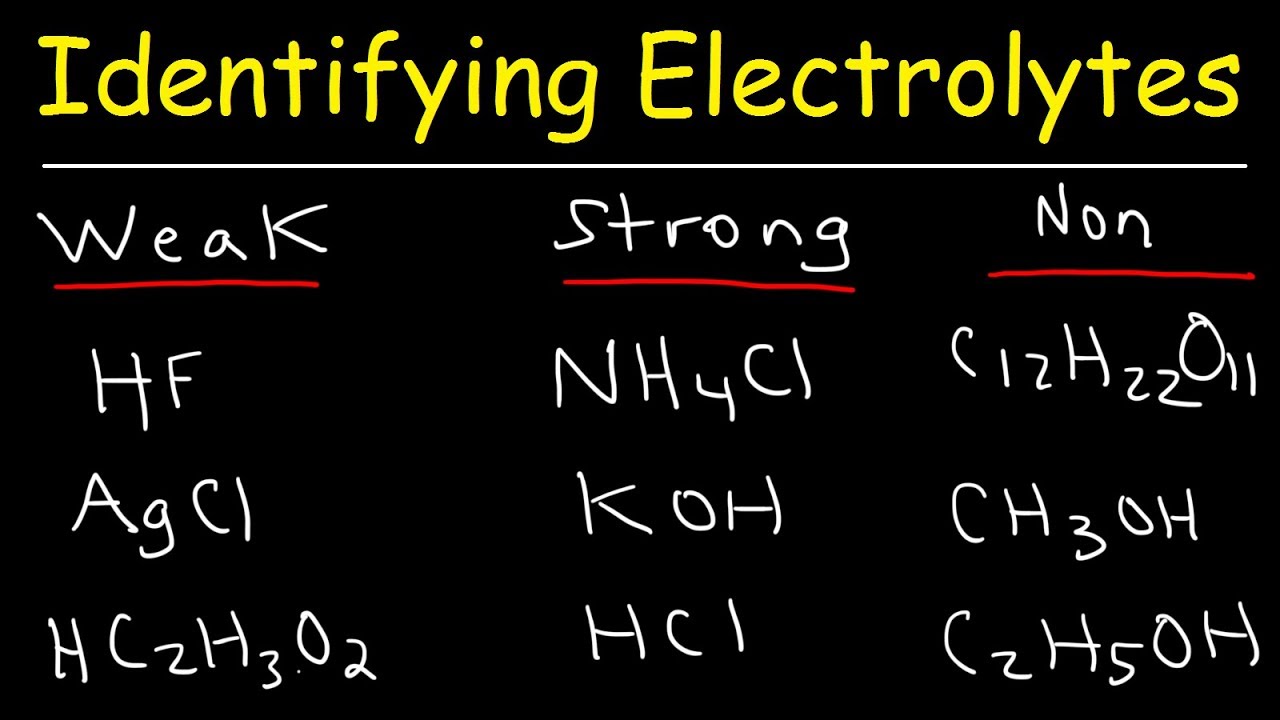
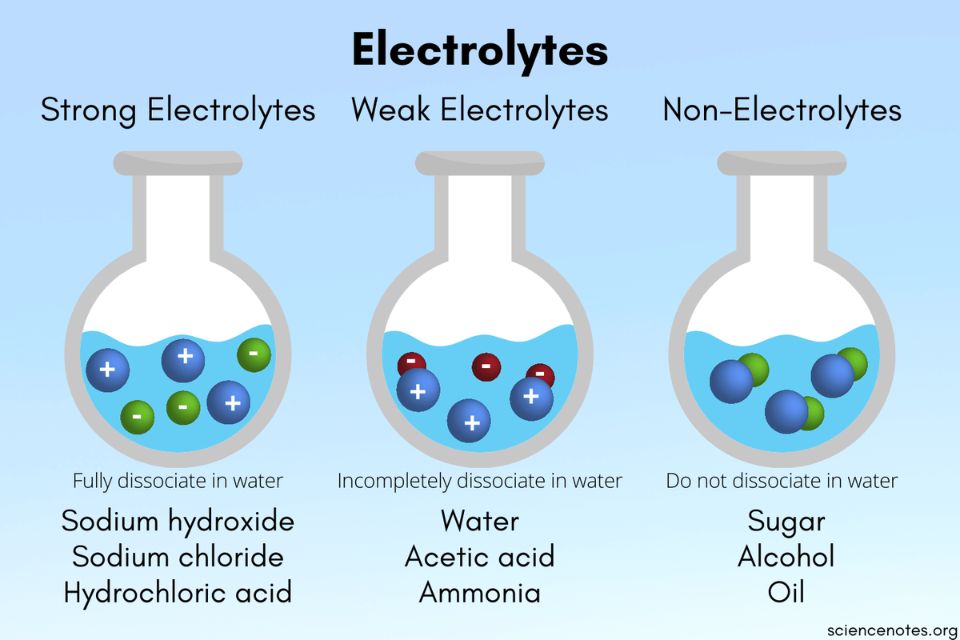
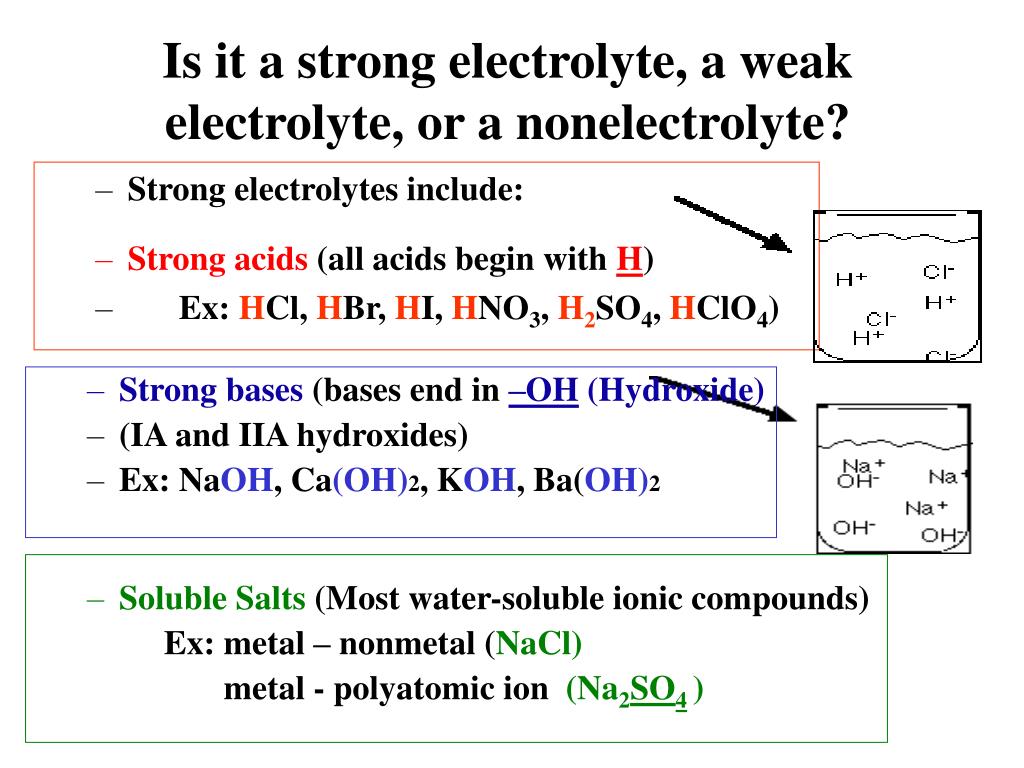
A salt that dissociates to a large extent is referred to as a strong electrolyte.
How to tell if a compound is an electrolyte. But there are strong acids that dissociate just as. A compound is an electrolyte if it dissociates into ions in solution and conducts electricity. Electrolyte balance is crucial to many body functions.
Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids. This simple guide aims to provide a clear understanding of the key characteristics that define. In order to conduct a current, a substance must contain mobile ions.
In general, it makes sense to guess that if the ions in a compound are very strongly attracted to each other, the compound will be less soluble, and also it might be. Electrolytes are important body constituents. One that does not dissociate much is called a weak electrolyte.
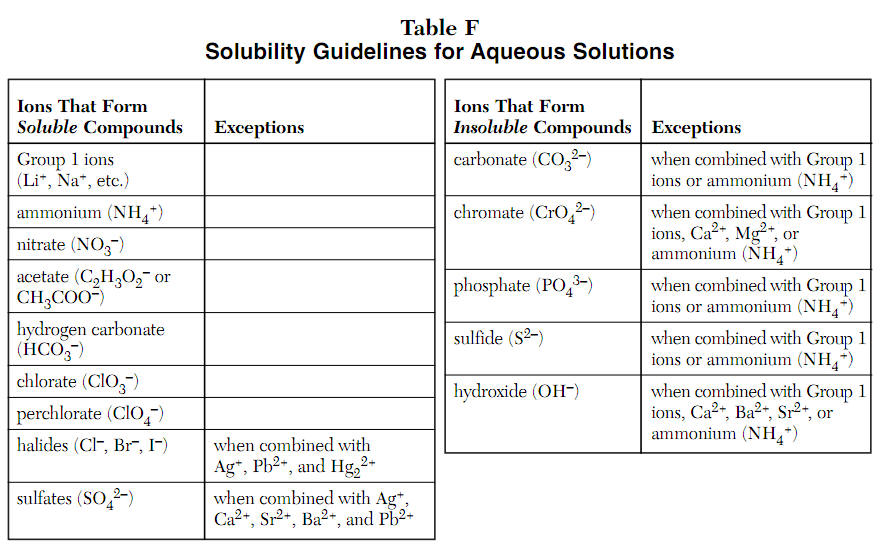
A nonelectrolyte is a compound that does not dissociate into ions in. Books put emphasis on mg+, na+, k+ as strong electrolytes. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity.
In order to conduct a current, a substance must contain mobile ions that can move from one electrode to the other. An electrolyte is a medium that contains ions and transmits electricity via ion movement. 1000 mmol = 1 mol and 1000 meq = 1 eq.
Ions can move in the liquid state (after melting) or in aqueous solution (after dissolving in water). Electrolytes are substances that dissolve in water to yield ions, such as strong electrolytes (ions that are easily dissociated) or weak electrolytes (ions that are not easily. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted.
Substances that dissolve in water to yield ions are called electrolytes. Electrolytes are just ions from a compound that you put in water. Here's some extreme examples of what can happen with an imbalance of electrolytes:
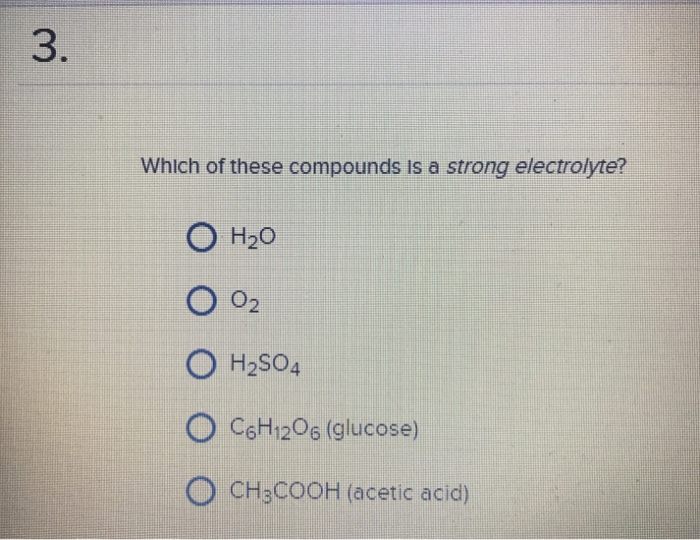
The electrolytes in the body fluids are usually reported in millimoles (mmol) or milliequivalent (meq) units, where: But how can you determine if a compound is an electrolyte? Learn the classification, examples, and difference between strong and weak electrolytes.
If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong. When ionic compounds dissolve, they break apart. Scientists can get some good clues as to the type of bonding in a compound by discovering whether a substance is an electrolyte or a nonelectrolyte.
All ionic compounds are electrolytes. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong.