Painstaking Lessons Of Info About How To Tell If It Is Polar Or Nonpolar
![[Solved] image attached 1. Complete the table below. Indicate whether](https://image1.slideserve.com/2610690/polar-or-nonpolar1-l.jpg)
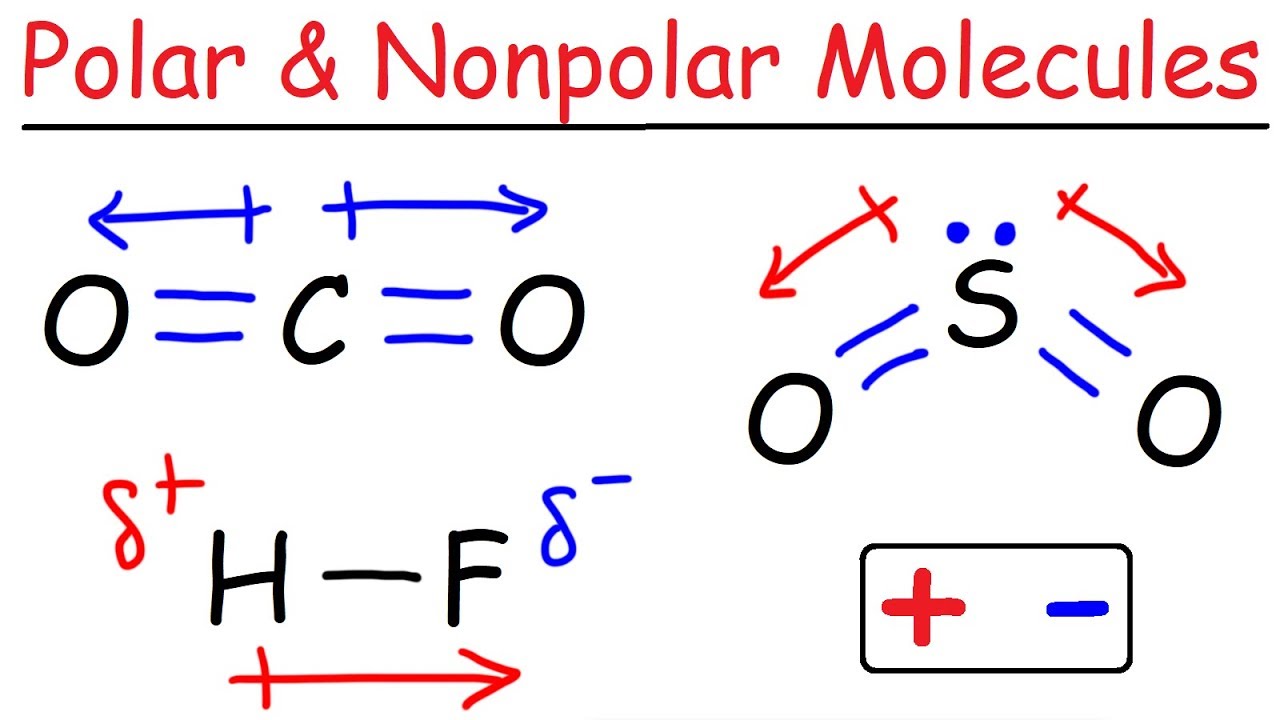
Like bonds, molecules can also be polar.
How to tell if it is polar or nonpolar. If different kinds of atoms are attached to the central atom, the molecule is polar. Polar molecules occur when there is an electronegativity difference between the bonded atoms. Check your score and answers at the end of the quiz.
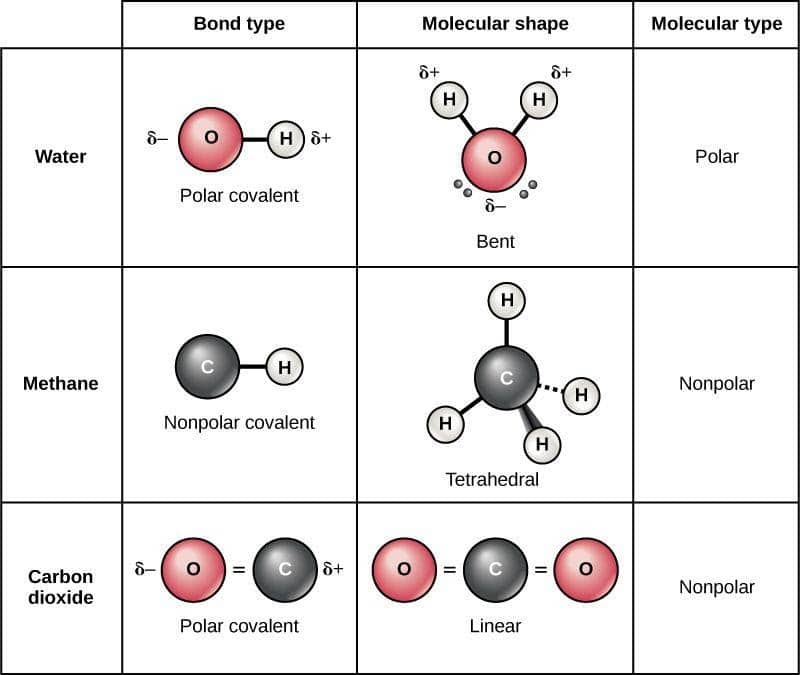
To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. This chemistry video tutorial provides a basic introduction into polar and nonpolar molecules. 4m views 10 years ago chemistry.
Does it have a polar covalent bond? Learn to determine if a molecule is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).we start with the polarity betwe.
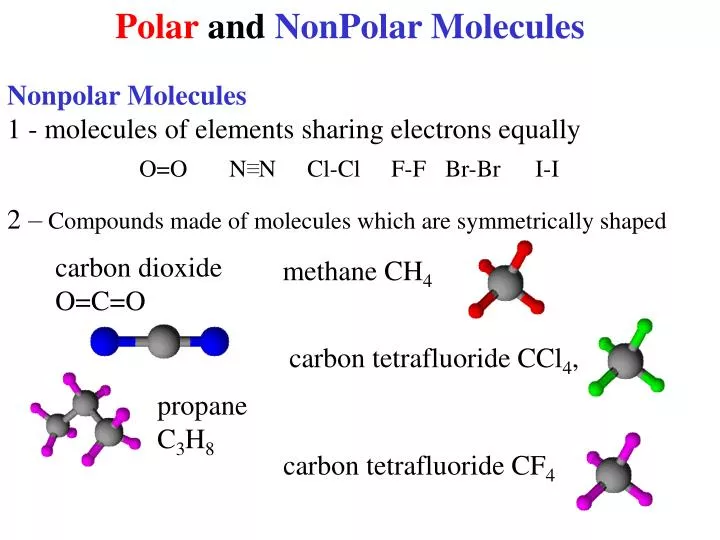
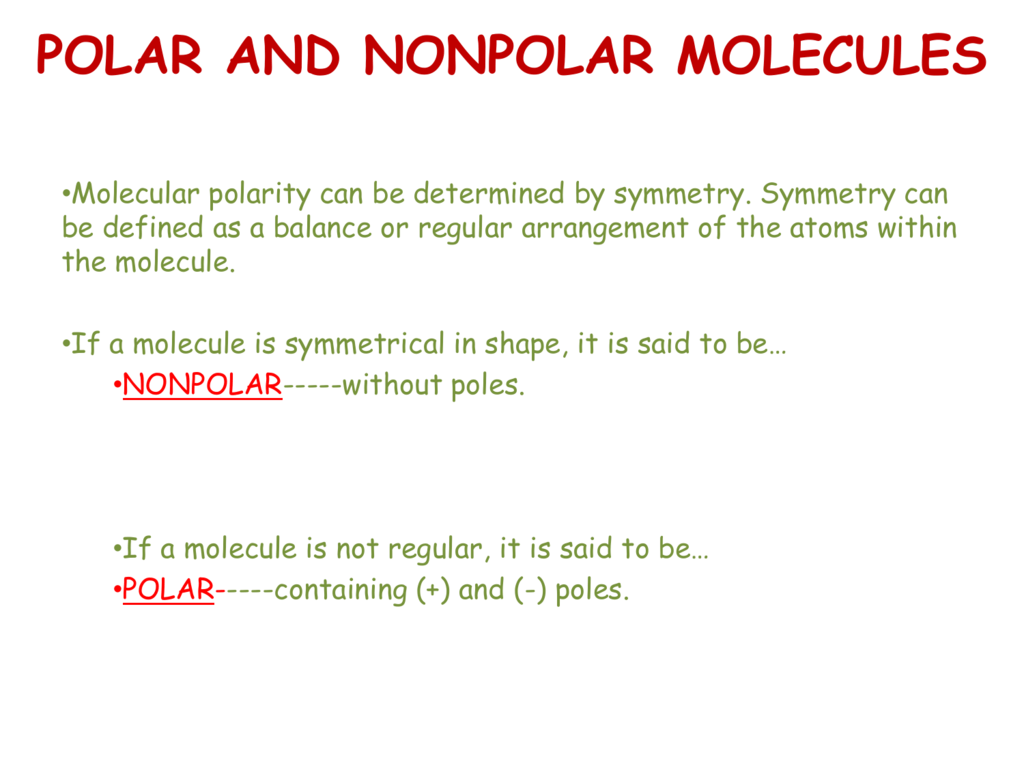
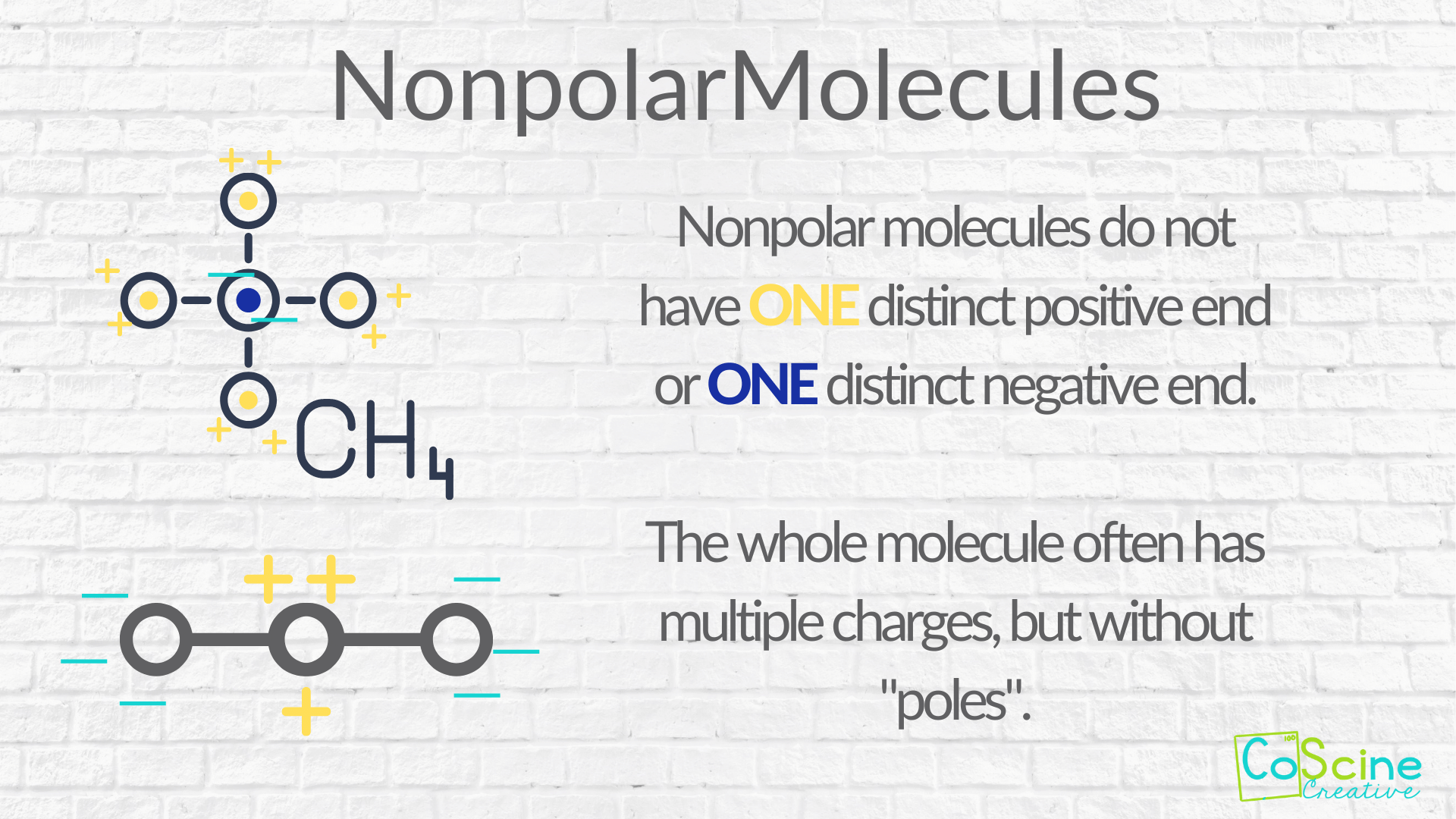
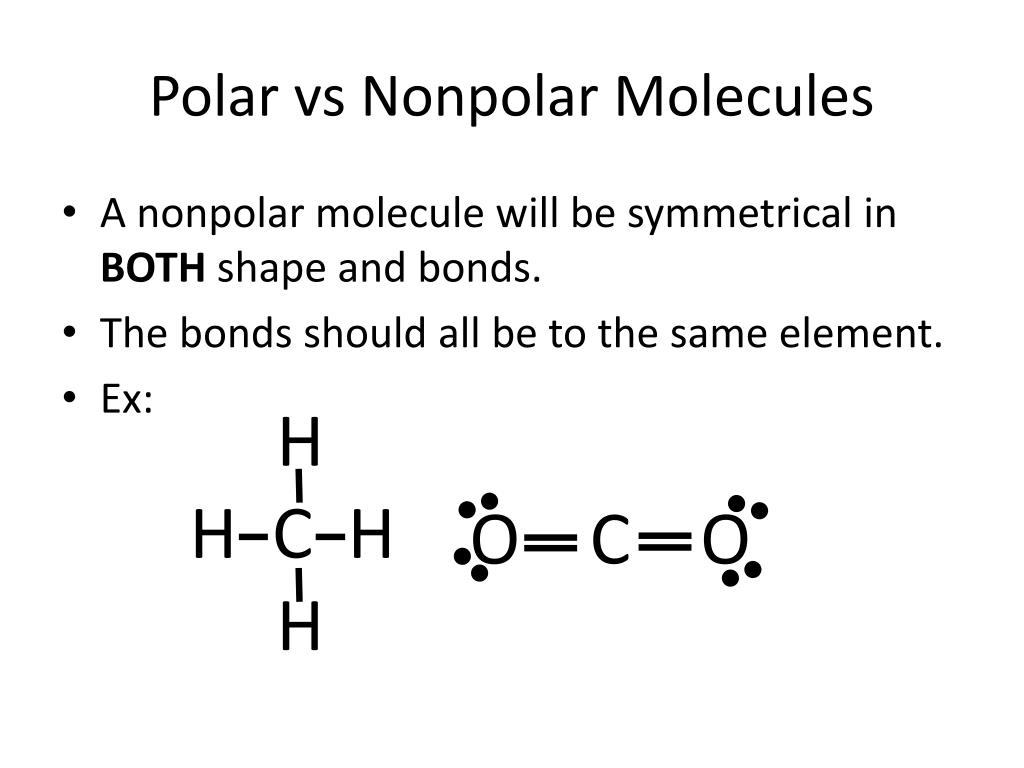
Nonpolar compounds will be symmetric, meaning all of the. The major difference between polar and nonpolar is polar bonds. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative.
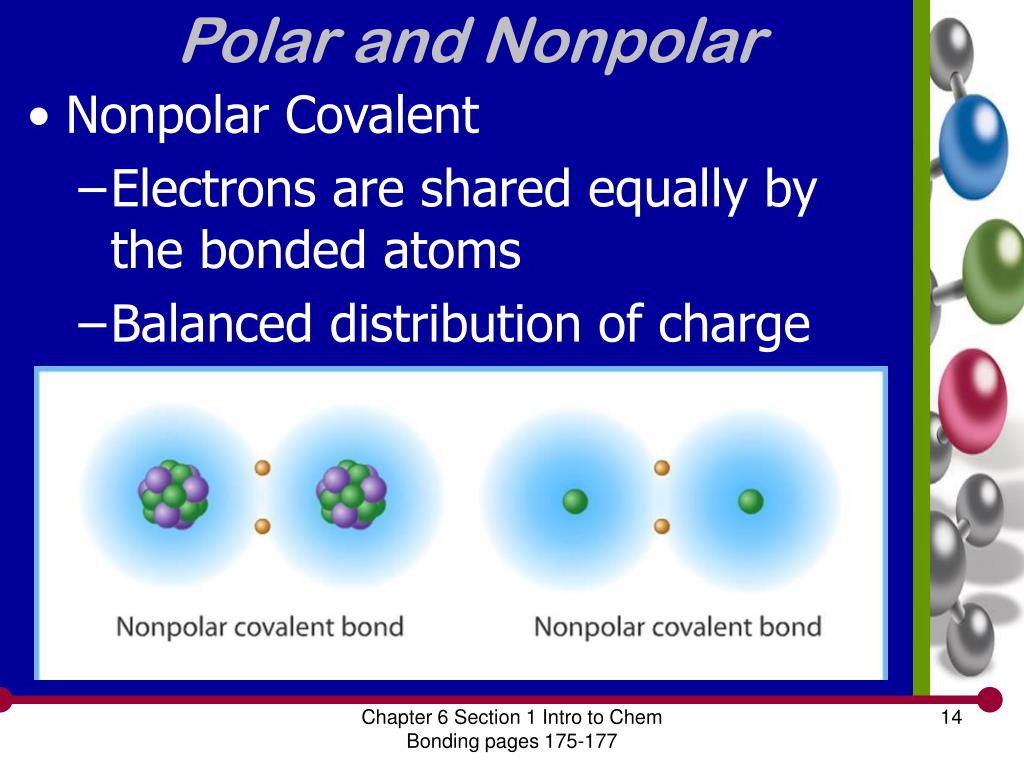
Select the correct answer and click on the “finish” button. A covalent bond that has an equal. Nonpolar molecules occur when electrons are shared equal.
It provides examples so you can quickly distinguish nonpolar molecul. Carbon tetrachloride has four polar covalent bonds. Are all bent molecules polar?
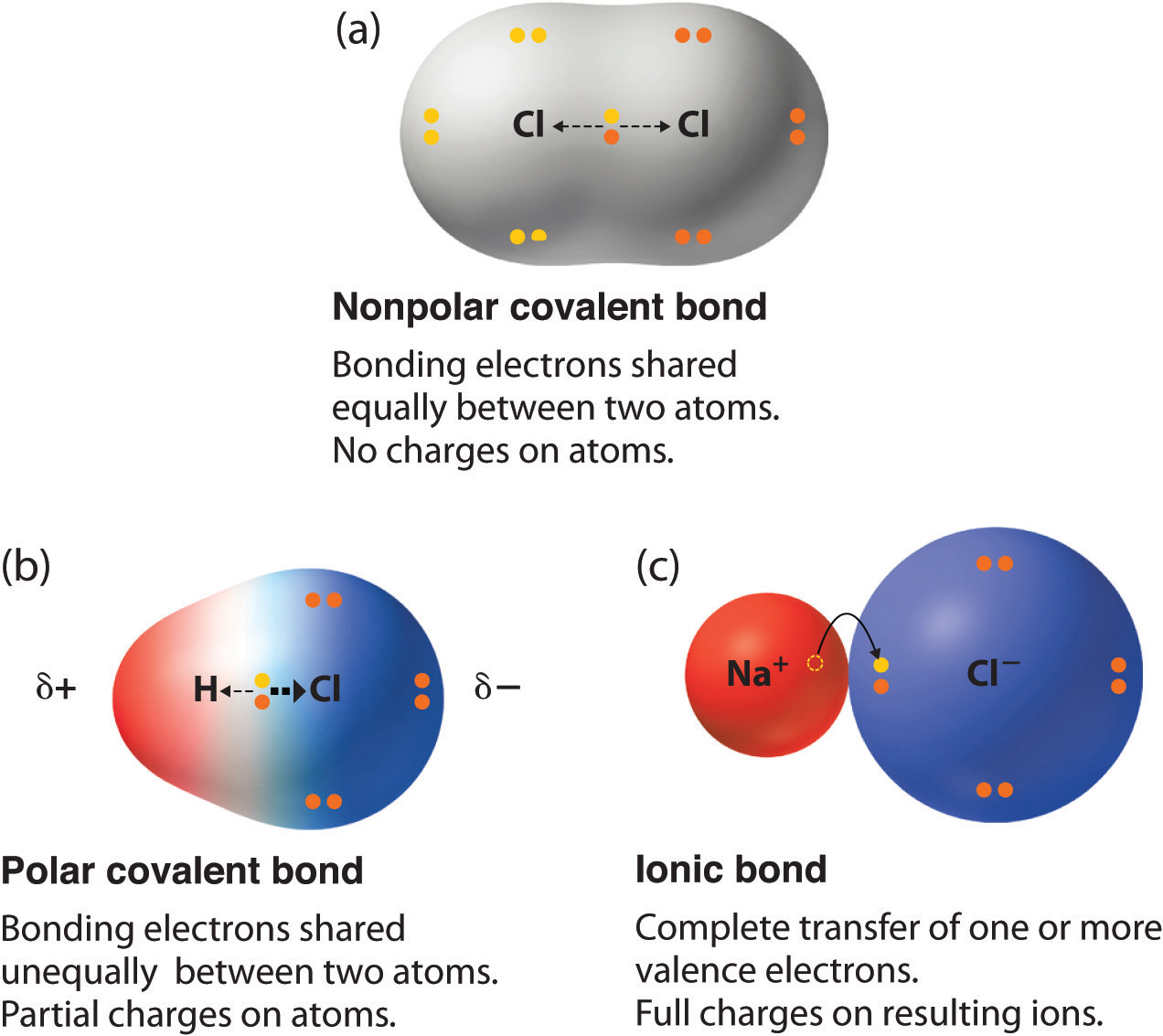
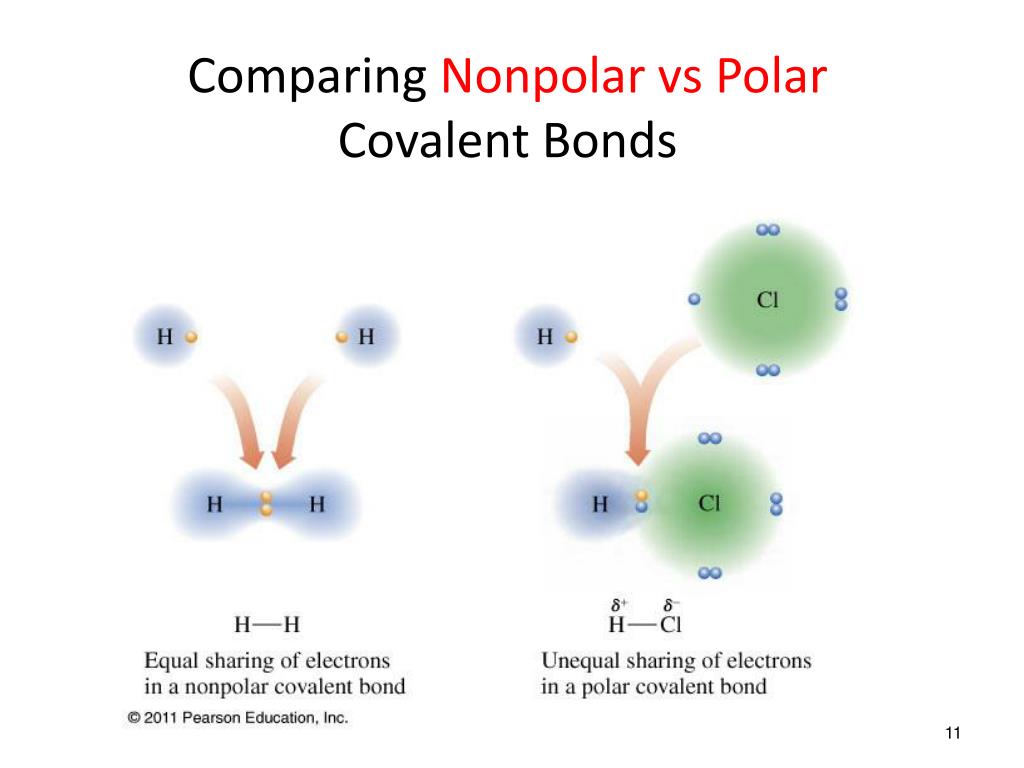
Determine if the bonds in the molecule are covalent or ionic. In the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. 1, is called a polar covalent bond.
To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. Find the relative electronegativity of the atoms in the molecule.
A completely polar bond occurs when one of the atoms is so electronegative that it takes an electron from the other atom (this is called an ionic bond ). A covalent bond that has an unequal sharing of electrons, as in part (b) of figure 4.4.1 4.4. As aforesaid, bent molecules are.
In this video, we're going to see how we figure out whether. This video provides a fast way for you to determine if a molecule is polar or nonpolar. Nonpolar compounds will be symmetric, meaning all of the.